The adoption of AI in Pharma has highlighted the growing need for innovative methods for increasing efficiency in the field. AI integration allows corporations to enhance employee training and education. Pharma eLearning platforms offer personalized and efficient training modules that cater to this highly specialized industry.
The 21 CFR Part 11 regulation, issued by the Food and Drug Administration (FDA), sets guidelines for electronic records and signatures in the pharmaceutical industry. 21 CFR Part 11 Compliance ensures the authenticity and confidentiality of electronic records and signatures. Failure to comply can result in severe consequences, including regulatory penalties and reputational damage.
This blog will explore how AI integration in Pharma eLearning can enhance industry efficiency and regulatory adherence. Pharmaceutical companies can streamline their training processes while ensuring compliance with the complex requirements of 21 CFR Part 11.
Through case studies, examples, and insights, this article will demonstrate the practical applications of AI in Pharma eLearning compliance.
Understanding 21 CFR Part 11 Compliance
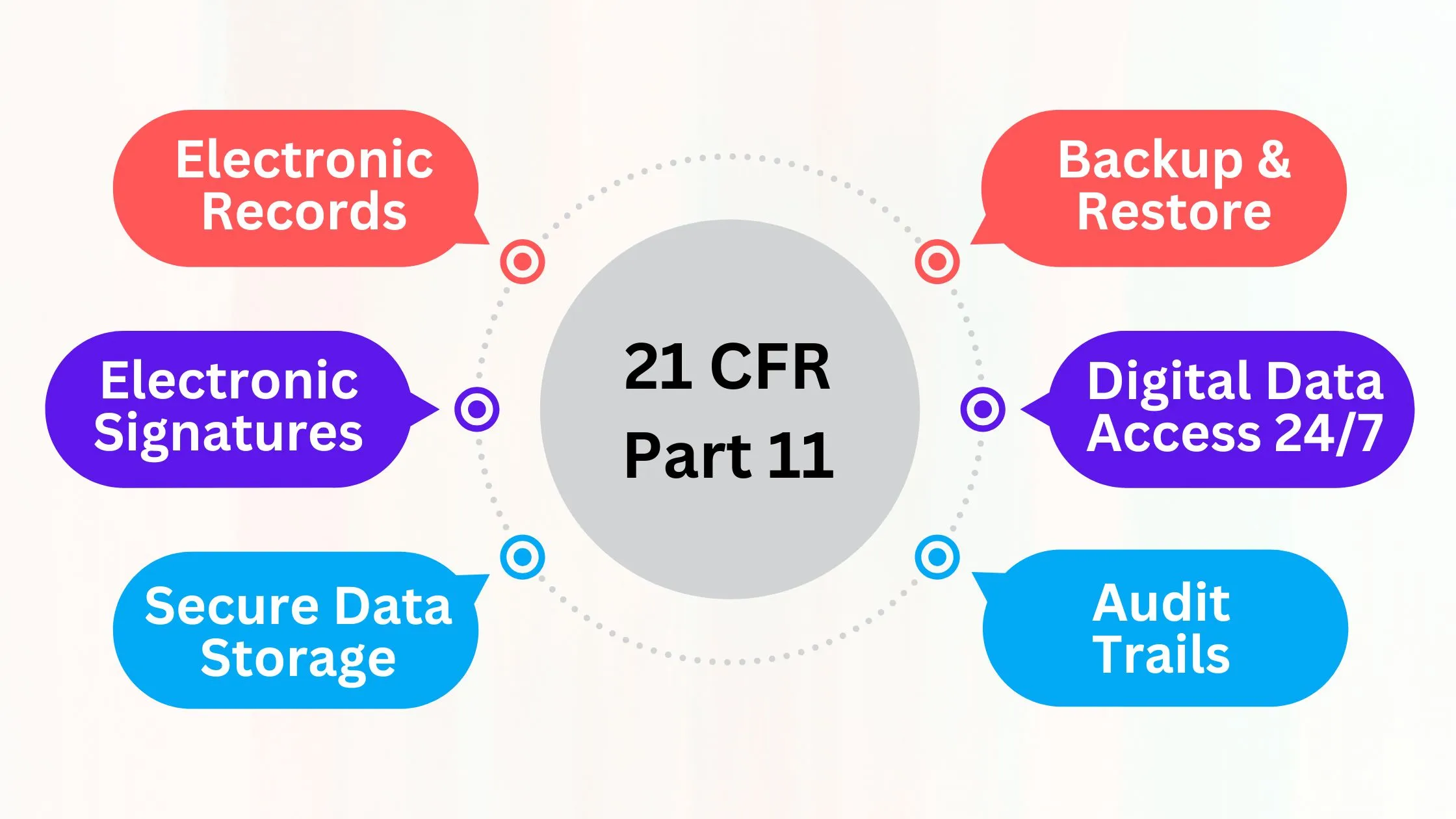
21 CFR Part 11 lays down foundational guidelines for electronic recordkeeping in the pharmaceutical industry. It ensures that electronic records and signatures are trustworthy and legally binding. Compliance regulations are necessary to maintain data integrity and ensure patient safety.
The requirements for 21 CFR Part 11 compliance include:
- Electronic signatures
- Access controls
- Audit trails
- Regular validation of electronic systems
These regulations prevent data tampering, ensure traceability, and enable standardized inspections by regulatory authorities.
Pharmaceutical companies face several challenges when ensuring compliance, including:
- The complexity of IT systems
- The need for robust data security measures
- The costs and time associated with validation
The rapid evolution of technology poses challenges in keeping up with changing Pharma training mandates and regulatory requirements. As AI advances, pharmaceutical companies must adapt their training programs to incorporate new technologies while staying aware of potential compliance gaps and patient safety risks.
Exploring the Role of AI in Enhancing Pharma eLearning
AI technology offers several advantages over traditional eLearning methods in the pharmaceutical industry. Personalized learning experiences adjust to individual needs, and the automation of repetitive tasks, such as content delivery and assessment, allows employees to focus on more resource-intensive activities.
Pharma eLearning enhancement involves the use of AI for process automation, such as:
- Content creation
- Material delivery
- Assessments
The automation of content creation and delivery streamlines the training process, which allows companies to scale their training programs to meet the demands of a growing workforce. AI-powered assessments provide immediate feedback to learners so that they can track their progress and identify areas for improvement.
By automating these tasks, companies can save time and resources while ensuring consistency and accuracy in training materials. AI-powered analytics provide valuable insights into performance and training efficacy.
AI algorithms can analyze learner behaviors, preferences, and performance data to deliver personalized learning experiences. Adapting the content and pace of training materials to individual learners allows automated eLearning platforms to improve engagement and knowledge retention. AI-driven eLearning platforms let learners engage in training activities at their own pace and convenience through mobile devices, desktop computers, or virtual simulations.
This personalized learning approach encourages a motivating learning environment where learning is more efficient.
Ensuring 21 CFR Part 11 Compliance with AI

AI tools can help pharmaceutical companies maintain compliance with 21 CFR Part 11 by automating different facets of recordkeeping and security. These tools automate the generation and management of electronic records, ensuring data integrity and authenticity. AI-powered audit trails and access controls help track and monitor user activities, facilitating regulatory adherence.
AI-powered eLearning platforms with robust tracking and auditing tools allow companies to monitor learner progress and compliance with the relevant training requirements. These platforms can generate detailed reports and audit trails, which can be invaluable during regulatory inspections.
AI solutions for compliance incorporate advanced security features, such as:
- Data encryption
- Access controls
- Anomaly detection
These comprehensive features safeguard electronic records and sensitive information. By implementing AI-powered data integrity and security measures, pharmaceutical companies can reduce the risk and impact of data breaches and ensure compliance with 21 CFR Part 11. Machine learning algorithms can analyze data to detect patterns of potential security breaches or abnormalities in user behavior, enabling immediate system intervention and resolution.
Pharmaceutical companies can use AI-powered data integrity and security measures to protect systems and uphold regulatory standards.
Case Studies: AI Success Stories in Pharma eLearning Compliance
Several pharmaceutical companies have successfully leveraged AI to enhance compliance with 21 CFR Part 11 in their eLearning initiatives.
For example, Pfizer utilized AI technology to analyze historical data and categorize adverse side effects experienced by patients using a Pfizer product during testing phases. With the automation of repetitive tasks, scientists can focus on more sophisticated tasks centered around patients. Evaluating information about adverse events for a vaccine or medicine is an essential aspect of pharmacovigilance and a regulatory requirement.
Such AI success stories highlighted by Pharma case studies exemplify the benefits of automation, including increased efficiency and accuracy. However, companies may also encounter challenges such as:
- Concerns over data privacy
- Integration issues
- Resistance to methodological changes
Despite these challenges, implementing AI solutions improves training effectiveness and compliance.
Based on real-world experiences, the best practices for effective AI integration in Pharma eLearning compliance include:
- Conducting risk assessments
- Proactively engaging involved parties
- Investing in employee training and management initiatives
Companies should continuously monitor and evaluate the performance of AI-powered eLearning platforms to ensure effective compliance implementation.
Future Outlook: The Evolution of AI in Pharma Training
The future of AI in Pharma eLearning looks promising with the continued advancements in technology and regulatory guidelines. As AI-powered eLearning platforms become more adaptive and personalized, they will cater to the changing needs of the pharmaceutical industry. Moreover, AI will address emerging challenges such as remote learning, skills gaps, and regulatory changes.
Several emerging trends and advancements are fueling the Pharma training evolution, such as:
- Implementation of natural language processing (NLP) for content generation
- Use of VR and AR for immersive training
- Integration of AI with technologies such as blockchain
AI integration in Pharma eLearning compliance has significant industry implications, including improved training effectiveness, regulatory compliance, and operational efficiency. It opens up new opportunities for growth and innovation as companies develop new training approaches and streamline product development.
By implementing the power of AI, the pharmaceutical industry can facilitate operations while maintaining proactive compliance.
Conclusion
As an AI integration recap, automation in Pharma eLearning has immense potential to enhance efficiency and compliance with 21 CFR Part 11. AI-powered eLearning platforms allow pharmaceutical companies to automate processes, personalize learning experiences, and ensure the integrity and security of electronic records.
AI will continue to shape the future of pharmaceutical training. Due to its inevitable expansion, the concept requires further exploration to refine the applications of AI technologies. Investing in AI integration will allow companies to thrive in the competitive pharmaceutical industry.