In industries such as healthcare, pharmaceuticals, and food manufacturing, meeting FDA compliance requirements is crucial to ensuring the safety, quality, and effectiveness of products. FDA compliance training is a pivotal element of this process. Leveraging an efficient Learning Management System (LMS) can significantly enhance how organizations handle FDA audits and manage compliance training.
This guide explores how an LMS can simplify FDA audits, optimize compliance processes, and ensure that employees are fully equipped to meet the requirements of the FDA.
What Is FDA Compliance Training?
Before diving into the technicalities of using an LMS to streamline compliance, it’s essential to understand the basics. FDA compliance training refers to the educational process that employees must undergo to ensure their activities align with the regulations set forth by the U.S. Food and Drug Administration (FDA). These regulations govern the manufacturing, labeling, distribution, and safety of food, pharmaceuticals, medical devices, and more.
Training typically covers topics such as:
- FDA regulations for product manufacturing
- Good Manufacturing Practices (GMP)
- Risk management
- Record-keeping standards
This ensures employees are equipped to perform their jobs in a way that supports overall regulatory compliance and quality assurance.
The Challenges of FDA Audits and Compliance
FDA audits are notoriously thorough and can be a significant challenge for many companies. The audit process includes examining your processes, systems, and training protocols to ensure compliance with FDA regulations. Failing to comply with these regulations can result in penalties, fines, or even product recalls.
Some of the challenges organizations face in maintaining FDA compliance include:
- Maintaining accurate training records
- Keeping up with frequent regulatory changes
- Ensuring consistent training across departments
- Demonstrating compliance during audits
These challenges can be time-consuming and labor-intensive, but an LMS can alleviate many of these issues by streamlining training management.
How an LMS Can Streamline FDA Audits and Compliance
An LMS offers an innovative approach to managing FDA compliance training and addressing the complexities of audits. Here’s how an LMS can simplify the process:
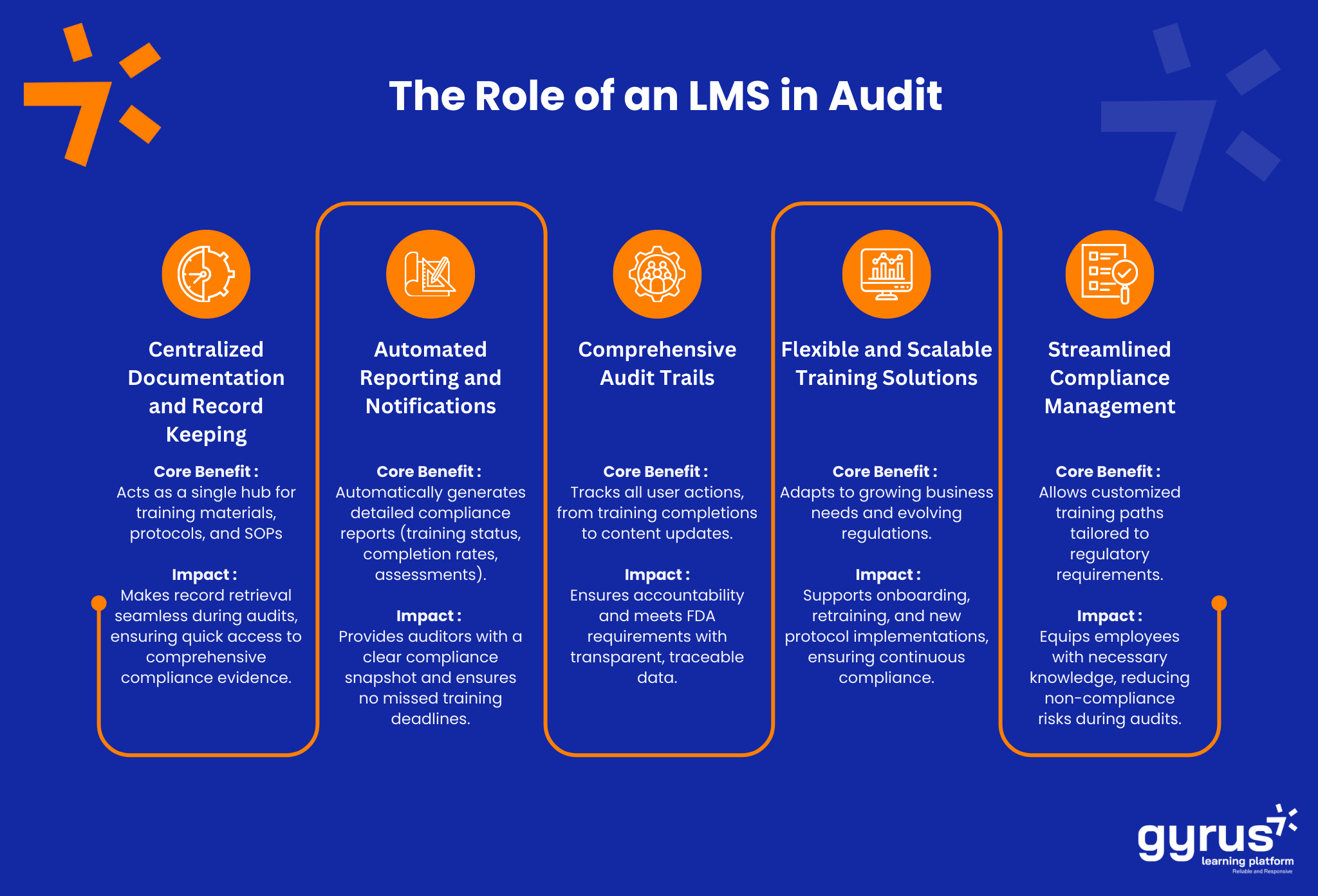
1. Centralized, Real-Time Training Records
When preparing for an FDA audit, one of the first things auditors look for is documented evidence that employees have received the proper training. An LMS centralizes training records, making it easy to track who completed which courses and when. Real-time updates ensure that all training is always current and relevant, which is especially important given the frequent changes in FDA regulations.
2. Automated Reporting and Tracking
Manually tracking training compliance is time-consuming and prone to human error. With an LMS, reporting is automated, and compliance tracking is seamless. The system will automatically generate reports that can be easily accessed and presented during an audit. These reports are crucial in proving that the organization is compliant with FDA training regulations.
3. Customizable Training Modules
Each department within an organization may require different training based on its specific responsibilities. An LMS allows for customized training paths tailored to individual needs. Whether it’s for quality assurance, manufacturing, or regulatory affairs, each team member will receive relevant training aligned with FDA compliance requirements.
4. Real-Time Updates for Regulatory Changes
FDA regulations often change, and keeping training programs updated is essential to ensure ongoing compliance. An LMS enables instant updates to training content, which can be rolled out across the organization immediately. This ensures employees stay informed about the latest FDA regulations, reducing the risk of non-compliance.
5. Interactive and Engaging Learning Experiences
An important challenge with FDA compliance training is keeping employees engaged. Traditional training methods may not be effective in ensuring that employees retain critical information. Modern LMS platforms include features like gamification, interactive content, and quizzes to engage employees, making training more effective and enjoyable. An engaged workforce is more likely to apply the training they’ve received, improving overall compliance.
Benefits of Using an LMS for FDA Compliance Training
Utilizing an LMS to manage FDA compliance training offers a range of benefits that extend beyond audits and regulatory adherence:
1. Time and Cost Savings
By automating and streamlining the training process, an LMS significantly reduces administrative costs and time spent on training logistics. The automation of scheduling, tracking, and reporting frees up resources for other essential tasks, contributing to improved efficiency.
2. Improved Training Consistency Across the Organization
As companies grow, maintaining training consistency across various locations can become challenging. An LMS ensures uniformity in training across departments, locations, or even global offices. Whether you’re operating in different regions or have multiple teams, an LMS ensures that everyone receives the same high-quality FDA compliance training.
3. Reduced Risk of Non-Compliance
FDA compliance training is a legal requirement for many organizations. An LMS helps reduce the risk of non-compliance by ensuring that employees receive the correct training and that records are easily accessible. The system provides clear evidence that training has been completed, helping organizations stay ahead of audits and inspections.
4. Efficient Tracking and Monitoring of Compliance
Using an LMS to track training ensures that no one falls through the cracks. You can monitor employee progress and completion rates, ensuring that everyone has undergone the necessary training and is up-to-date with FDA compliance requirements. This is especially crucial during an FDA audit, where proof of training must be readily available.
Key Features to Look for in an LMS for FDA Compliance
Not all Learning Management Systems are created equal. To ensure that your FDA compliance training is effective, look for an LMS that offers the following key features:
- Audit Trails: Ensure that your LMS has the ability to track user activity and generate detailed audit trails.
- Mobile Access: Mobile compatibility ensures that employees can access training content on the go, which is especially helpful for field staff.
- Compliance Certifications: An LMS that issues compliance certifications helps to confirm that employees have completed required training.
- Integration with Other Systems: Seamless integration with other compliance management or HR software can improve the overall management of compliance.
Conclusion: Enhancing FDA Compliance and Audit Readiness with an LMS
An LMS offers a strategic advantage in managing FDA compliance training and preparing for FDA audits. By centralizing training records, automating reporting, and delivering consistent training, an LMS can help organizations stay compliant with FDA regulations while also reducing the time and effort needed for audits. When you invest in the right LMS, you not only streamline your FDA compliance processes but also ensure that your organization is always prepared for any audit, keeping operations smooth and compliant.









